Hardly a month goes by without new research findings that may ultimately change our approach to treating breast cancer. Just yesterday, researchers revealed that the cells giving rise to hereditary breast cancer resulting from the BRCA1 mutation can be targeted with an anti-osteoporosis drug called denosumab. This raises the possibility that this monoclonal antibody might actually prevent breast cancer in these women and save them from having to undergo prophylactic mastectomies. However, this approach, although exciting and on solid scientific ground, will need to be carefully assessed in properly-designed clinical trials to know whether it works and is safe over the long term. If so, it will almost certainly become the new approach in this select group of women with a very high lifetime risk of developing breast cancer.
The BRCA1 story reminds me of an important study, called METABRIC, published in 2012, that found there were ten different types of breast cancer. I reported on it in my CTV.ca/health blog, re-posted below.
The BRCA1 story reminds me of an important study, called METABRIC, published in 2012, that found there were ten different types of breast cancer. I reported on it in my CTV.ca/health blog, re-posted below.
Improving the treatment of breast cancer: A tale of 10 diseases
by Dr. Lorne Brandes April 23, 2012
Having treated breast cancer for four decades, I can attest to the progressive improvement in outcome in women diagnosed with early-stage disease. Multi-pronged advances in chemotherapy, radiation and hormonal therapy, aided by earlier detection through regular screening mammograms, have increased the five-year survival rate from 71% when I began practice in the early 1970’s, to 90% in 2012.
Yet, despite this impressive gain, and beyond what the statistics tell us, I am always humbled by our inability to predict the outcome for any specific patient. I remember the faces of those I thought would do well but didn’t, and, conversely, of those I thought might fare poorly but did well.
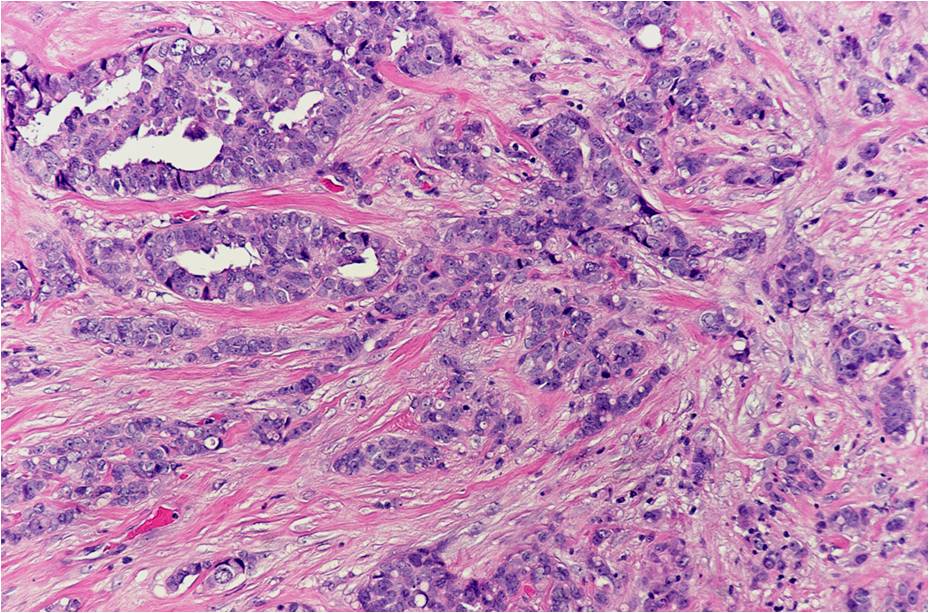
The reason? Despite the fact that two women may present with breast cancers of identical clinical stage (the degree of spread) and pathological grade (the degree of malignancy under the microscope), their tumours may have radically different biological characteristics and sensitivity to treatment.
For example, approximately two-thirds of malignant breast tumours contain receptors for the female hormones estrogen and/or progesterone. A high content of both hormone receptors generally indicates a better prognosis; recurrence often can be prevented by oral antiestrogen drugs such as tamoxifen or the newer class of aromatase inhibitors. On the other hand, the one-third of tumours that lack hormone receptors are less predictable in their behaviour; nonetheless, chemotherapy significantly improves the outcome in such cases.
Twenty per cent of breast cancers produce high levels of a receptor protein called HER2 . These tumours usually lack estrogen and progesterone receptors, tend to be very aggressive ( high grade) and are more likely to spread (metastasize). However, in recent years, their recurrence rate has been cut in half using chemotherapy in combination with the HER2-targeting drug, Herceptin.
Tumours that lack estrogen, progesterone and HER2 receptors are called triple-negative; they also tend to be high grade. Unfortunately, they often recur despite aggressive chemotherapy and radiation, but those that do not come back within 2 to 3 years after treatment are usually cured.
While tailoring breast cancer therapy according to stage, grade, and receptor status has greatly improved overall survival, choosing the best treatment in each case, based on an accurate prediction of individual outcome, has remained elusive.
But now, that situation is about to change dramatically.
As a result of a landmark study just published online in Nature, we have been given a whole new roadmap of the biology of breast cancer; with it comes the ability to more accurately predict the behaviour of individual tumours.
This breakthrough was made by a consortium (known by the acronym METABRIC) of Canadian- and U.K-based. cancer researchers led by Drs. Samuel Aparicio of Vancouver’s British Columbia Cancer Centre and Carlos Caldas of the Cambridge Research Institute.
By analyzing the DNA in almost 2,000 breast cancer tumours, the scientists discovered that individual tumours contain one of 10 different “clusters” of altered (abnormal) genes. Some of the genes (like the one that produces the HER2 protein) were predictable, but several others had no previously known link to breast cancer.
The group’s conclusion? Breast cancer is not one, but 10 different diseases! Each subtype exhibits unique genetic (and, therefore, biological) characteristics.
But there is more. Because the outcome of treatment was documented in every tumour donor, the prognosis for each of the 10 subtypes of breast cancer is now known!
As one example, tumours with altered “cluster 4” genes, including some high-grade triple-negative cancers, were observed, surprisingly, to be associated with a good prognosis. Intriguingly, cluster 4 alterations do not involve cancer genes; rather, they are associated with immune system genes. This might explain why a rare form of high-grade triple-negative breast cancer, called “medullary”, has a better than average prognosis: medullary breast cancers are typically infiltrated by millions of “killer” immune cells, called CD8 lymphocytes.
These findings raise the possibility that “cluster 4” immune system genes could be harnessed to improve the outcome in other, less responsive forms of breast cancer.
To illustrate, although estrogen receptor-positive breast cancers generally have a good prognosis, the METABRIC study identified two exceptions: an estrogen receptor-positive subtype, containing “cluster 2” gene abnormalities on chromosome 11, responds poorly to blocking estrogen; a second estrogen receptor-positive subtype, containing “cluster 5” abnormalities on chromosome 17 (the HER2 gene), has an extremely poor prognosis despite the use of chemotherapy, Herceptin and antiestrogens.
Could immune stimulation change the poor outcome in these two instances? Evidence that this could be so comes from recent studies showing that chemotherapy given prior to surgery can eradicate breast cancer and significantly increase survival when CD8 cells infiltrate tumours in high numbers.
Commenting on the importance of the METABRIC study, leading British genetics researcher, Professor Charles Swanton, described the new results as an “extraordinary” finding that took our understanding of breast cancer “to the next level”. These findings are “likely to have important implications for clinical trial design in breast cancer and will prime researchers worldwide to define new approaches to treat each subgroup,” he said.
Lead METABRIC researcher, Carlos Caldas, added, “I want to stress, this…wouldn’t have been possible without the breast cancer patients who donated their samples and agreed to take part in the study. None of this would have happened without them, and I’m so grateful for their participation.”
My take? While the full impact of these findings will take years to realize, they are truly revolutionary, certain to ultimately result in more effective breast cancer treatment and improved survival.
 RSS Feed
RSS Feed
