With the recent death of Muhammad Ali from complications of Parkinson's Disease (PD), new attention has turned to the causes and treatment of this all-too-common neurodegenerative disorder. As a result, I am re-posting a blog I wrote for CTV.ca/health in 2012 that examined the role of iron buildup in the brain that may promote the formation of an abnormal prion-like protein, called alpha-synuclein, that many neuroscientists now believe to be at the root of PD and may explain the progressive spread of this disease throughout the brain over time. The blog garnered much interest and many citations on other websites. Here it is.
Targeting iron in Parkinson’s Disease
by Dr. Lorne Brandes Jul. 4, 2012
There are some exciting findings afoot to suggest that too much iron in brain tissue is a common finding in neurodegenerative diseases, and that removing it with certain drugs, called chelators, may significantly improve symptoms.
But to help you understand this, I first need to review some additional scientific information.
In a recent blog on Alzheimer’s Disease (AD), I reported on a misfolded protein, called tau. In its normal form, tau has a corkscrew appearance (called an “alpha-helix”). However, when too much tau is made by cells, multiple tau molecules stick together (aggregate). In the process, tau changes shape (misfolds), becoming a pleated structure (called “beta sheets”) that is toxic to nerve cells.
Of great interest, pleated tau structurally resembles a prion, the infectious agent that is best known for causing mad cow disease (BSE, or bovine spongiform encephalopathy). BSE can be transmitted to humans who eat prion-infected meat (the human form of BSE is Creutzfeldt-Jakob disease, a rapidly-progressive neurodegenerative disorder).
Even if tau looks like a prion, does it behave like one ? It appears that it does: researchers have discovered in mice that, similar to the progression of AD in humans, tau first accumulates in a memory area of the brain called the entorhinal cortex and then spreads along nerve networks “like an infection” to progressively involve other memory centres.
How does pleated tau spread? Scientists believe that it uses itself as a template to form replicas that leave the cell and move from nerve to nerve. That said, unlike BSE, so far there is no evidence to suggest that tau can survive outside the nerve cells of the human brain or be transmitted from one person to another.
Yet, transmissible or not, the prion-like structure and “contagious behaviour” of tau has suddenly become a hot topic among neuroscientists who quickly point to another misfolded (pleated) protein, called alpha (α)-synuclein, a neurotoxin that is strongly linked to Parkinson’s Disease (PD).
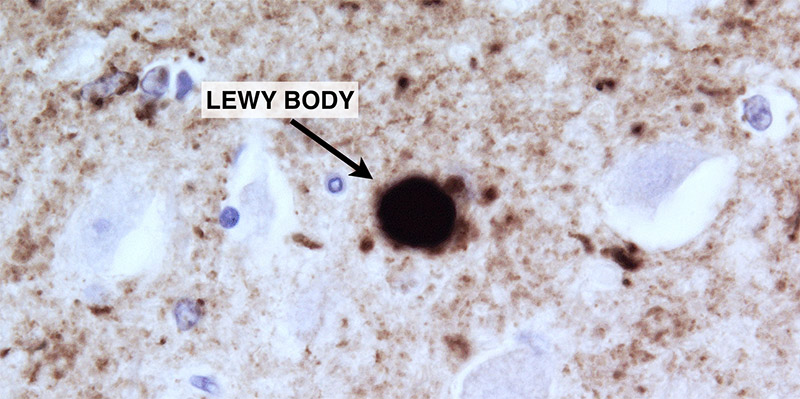
Under the microscope, nerve cells affected by PD contain rounded inclusions, called Lewy bodies (named after their discoverer, Dr. Frederic Lewy), that are rich in alpha-synuclein. Pathologists have also identified Lewy bodies in human embryonic stem cells that were implanted into brains years earlier in studies to treat PD.
Noting that these transplanted donor cells must have become “infected” with alpha-synuclein from the recipients’ brains, Dr. Stanley Prusiner, who won the 1997 Nobel Prize for discovering the prion, recently suggested that, indeed, PD may be “a prion disorder”!
Is he overstating the case?
Consider this: we now have evidence that, even before PD affects the brain, it starts in the sympathetic nerve fibres that control bowel function. Researchers have found biopsy evidence of alpha-synuclein in gut tissue up to five years before typical brain-related symptoms of tremor and muscle rigidity occur. Indeed, chronic constipation, likely caused by alpha-synuclein damage to sympathetic nerve cells, may be the earliest symptom of PD.
Over time, the disease appears in (spreads to?) the brain, initially involving the brain stem, and then moving to an area of dark-staining brain tissue, called the substantia nigra (SN).
The cells of the SN produce a neurotransmitter chemical, called dopamine, that is vital to normal brain function. When approximately 80% of the dopamine-producing cells die from the effects of alpha-synuclein, the typical tremor and/or rigidity symptoms of PD appear.
Although a drug called L-dopa can relieve these distressing symptoms by replenishing the lost dopamine, it is by no means a cure. Nerve cells continue to wither from alpha-synuclein as the disease spreads to other areas of the brain and spinal cord.
Now (and thank you for being patient) here is where iron comes into the picture: in addition to alpha-synuclein, Lewy bodies contain abundant iron. In addition, a heavy buildup of iron is found specifically in brain areas affected by PD.
Perhaps most important of all, new studies show that iron not only regulates cell production of alpha-synuclein, but promotes its misfolding into the neurotoxic prion-like pleated form!
Suddenly, many researchers are suggesting that removing the buildup of iron in brain tissue might be one way to control the production (and, possibly, misfolding) of alpha-synuclein, thereby slowing or halting the progression of PD and other neurodegenerative diseases.
Indeed, early clinical trials of an FDA-approved oral iron chelator, called deferiprone, have shown promise to decrease brain iron and relieve symptoms in a related neurodegenerative disease called Friedreich’s Ataxia (spinocerebellar degeneration).
Could deferiprone also benefit patients with PD? Dr. David Dexter of London’s Imperial College hopes to find out. He is the principal investigator of a phase 2 trial involving 36 patients; the study, which opened in February, has already accrued close to half of the required subjects.
If the results look promising, phase 3 studies in PD, as well as new trials in other neurodegenerative diseases where brain iron levels are high, such as Huntington’s Disease and Lewy Body Dementia will almost certainly follow.
But as always, there is a note of caution. Even if iron chelation therapy helps patient with PD, it is unlikely to be curative. Nerve cells that have already succumbed will not return. Alpha-synuclein that is already misfolded might continue to act as a template, spreading the disease. Dopamine will still need to be buttressed by drugs like L-dopa. Moreover, deferiprone has some potentially serious side effects that must be weighed against any potential benefits.
Nonetheless, the tantalizing prospect that, through its effects on alpha-synuclein, iron may play a major role in more than one neurodegenerative disease process, and that chelation therapy could be an effective antidote, is worth everyone’s attention
 RSS Feed
RSS Feed
